It can have escaped few of us that the effects of the war in Ukraine and the fallout of the COVID-19 pandemic, alongside the ongoing issue of climate change, have led to renewed interest in the possibilities offered by nuclear power and how it can help to solve the problems governments around the world are facing. This is leading to a re-evaluation of the case for nuclear, and, hopefully, objective consideration of its strengths and weaknesses.
I believe that it is essential that Western governments rethink the strategic role it can play in the domestic energy sector. Over a series of articles, we’ll discuss the technology, the dangers and the accidents, and talk about the limitations of other renewable forms of energy.
Let’s start first with how it works. Before we begin, we can look at how fossil fuel functions, as a comparator. Fossil fuels such as oil, coal and gas incorporate energy that originally arrived from the sun. Plants store the sun’s energy via photosynthesis to convert carbon and oxygen from the air, plus hydrogen extracted from water, into carbohydrates and other substances. Pressure, heat, and time – over millions of years – convert these substances into hydrocarbon fuels, such as peat, lignite and coal. Oil and gas deposits are generally found where there was once water-borne life. When we burn hydrocarbon fuels, we reverse the process : when heat is introduced, the hydrocarbon molecules are split, reacting with oxygen in the air to produce water and carbon monoxide/dioxide gas.

Nuclear energy works in a completely different way, and to understand it we need to look at the physics around the building blocks of matter itself, discovered and refined during the 20th century. It was found that elemental matter – including the carbon, oxygen and hydrogen as well as metals and other substances that we are made of – consist of atoms, which in turn are made up of subatomic particles, namely protons, electrons, and neutrons. The protons and neutrons form the atom’s nucleus, while the electrons sit at a distance (they are commonly shown in orbit of the nucleus although this is not quite accurate). These particles are in turn composed of smaller elements – quarks – an ongoing topic of research in particle physics.
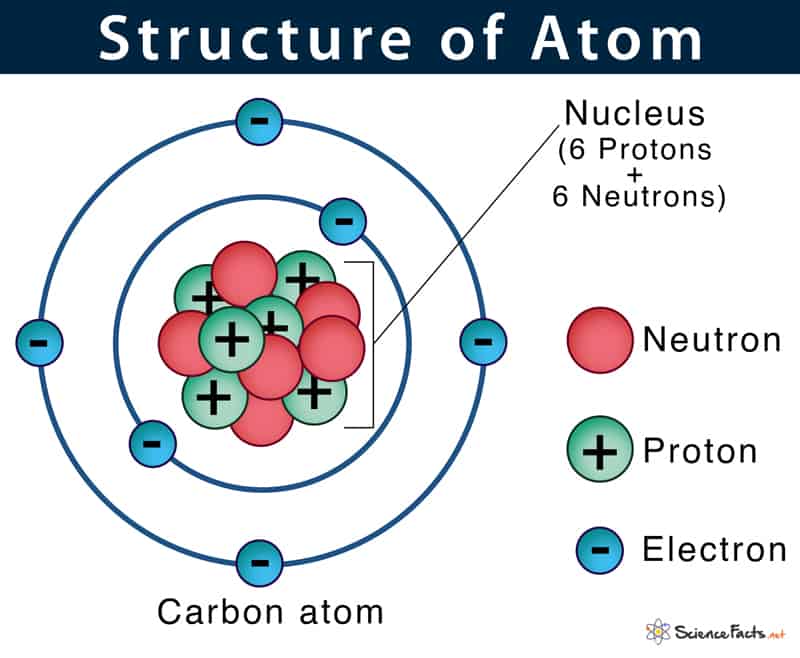
The simplest and most plentiful elements in the universe are hydrogen and helium, which consist of one and two protons respectively and a variable number of neutrons (each variation in the number of neutrons is known as an isotope). Enormous amounts of energy and confinement are required to push protons and neutrons together to make more complex elements. These conditions exist within stars. Everything that we and our surroundings are made of – carbon, oxygen, iron, calcium and many dozens of other elements – was created by nucleosynthesis either within a star; when a star exploded; or within the leftovers of an exploded star.
The number of protons and electrons governs the chemical/electrical properties of an element and how it reacts with other elements. The number of neutrons has no impact on the element’s chemical properties. However, some isotopes are unstable, releasing small numbers of particles and energy spontaneously. Everyone has heard the word which describes this process : radioactivity. There are three major types of radioactivity – alpha and beta, which release particles moving at speed, or gamma, which is electromagnetic energy. Radiation can change other atoms or molecules, triggering biological cells to self-destruct, which is why it is dangerous to life. Following an alpha or beta emission, the atom becomes another element which may be more radioactive, less radioactive or completely stable. It’s not possible to predict exactly when a given atom will emit radiation, but the number of emissions over a period of time – the half life – is known. Some isotopes have half lives of tiny fractions of a second; others are billions of years. Shorter half lives are more dangerous as there are more decay events going on at any given time. The greatest danger comes from elements which are absorbed by humans, such as radioactive iodine (absorbed by the thyroid gland, triggering thyroid cancer) or strontium (absorbed like calcium into bones). Both of these elements are produced in nuclear reactors and by nuclear weapons. This is why iodine tablets are issued in the event of a nuclear incident, to ensure the radioactive iodine does not get absorbed.

Aside from radioactive decay, there is another way that a nucleus can be made to eject particles and energy. The largest combinations of protons and neutrons – such as uranium and thorium – can also be triggered to split into several (typically two or three) chunks when struck by a neutron moving at a specific speed. This is called nuclear fission and the substances which lend themselves to this are known as fissile isotopes. It forms the basis for all commercial nuclear reactors operating today, as well as forming a key part of a nuclear bomb. The amount of energy released during fission is millions of times the amount released when the hydrogen and carbon atoms in hydrocarbon fuel split from each other. Each fission event will result in the creation of fission products : different elements which are often themselves highly radioactive. These fission products constitute high-level nuclear waste, and pose a significant challenge that needs to be considered as part of the overall case for nuclear power.
The diagram below shows a uranium-235 nucleus being struck by a neutron. In this case it splits into barium-144 and krypton-89, both of which are radioactive. Three neutrons are released, which can go on to split more uranium nuclei.

As our understanding of these physical processes grew during the 20th century, attention turned to how these energy sources could be harnessed, both as weapons and for civilian applications. This required a way to make uranium release energy at a steady and controlled rate. It was soon discovered that a uranium fission event, triggered by a moving neutron, would release more neutrons which could in turn trigger more fission events, creating a self-sustaining reaction. However, the neutrons emitted during fission move too fast to trigger further fission. Therefore they must be slowed down by bouncing off other atoms, within a substance known as a moderator. To stop the reaction, all you need to do is get rid of the neutrons by absorbing them within another, non-fissile substance.
A nuclear reactor is therefore, conceptually, quite a simple device. In the same way that a fire requires fuel, oxygen, and heat, a nuclear chain reaction requires a fissile substance, neutrons, and a moderator.
In practice, most modern nuclear reactors are pressurized water reactors, which are essentially a very large, thick steel drum, roughly the size of a large truck or lorry. The reactor is filled with water, which serves as both a coolant and a moderator. The nuclear fuel is arranged as a series of rods within the reactor vessel. The water pressurized within a closed loop and pumped through this array of rods, carrying away the heat produced by the reaction to generate steam to generate electricity via turbines, while also providing the moderation necessary to maintain the reaction. This provides a degree of safety – if the water gets too hot, it expands, which reduces its moderation capability and causes the reaction to slow.
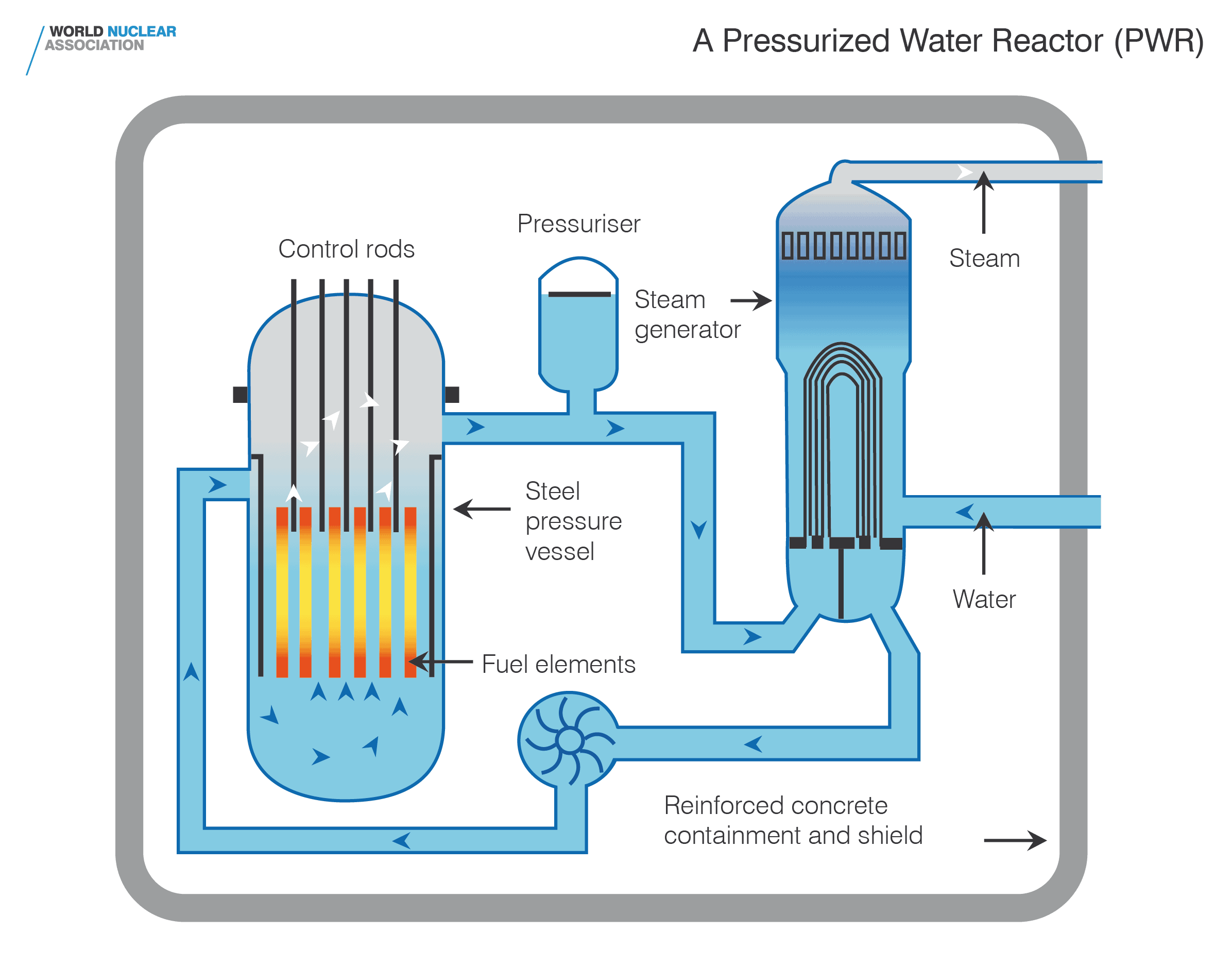
The particular properties of nuclear reactors lead to a number of important characteristics. Where a fossil fuel station needs to be continuously supplied with gas, coal, or oil, a nuclear reactor runs typically for around 18 months without having to be refuelled and can, in an emergency, be operated for longer still. When the time comes to refuel a pressurized water reactor, it must be shut down for several weeks. During this outage, the reactor is partially dismantled, submerged under water and inspected. Since fission occurs at different rates within the reactor vessel, the fuel rods are moved around within the reactor over a number of refuelling cycles before they are considered spent. A fuel rod can stay within a reactor for anywhere up to five years before being replaced. When exhausted, the contents of the fuel rod are still highly radioactive and emit quite a lot of heat, so must be moved to a cooling pond for a few years for the radioactivity to die down.
The image below shows a reactor with the head removed, looking down at the fuel rods undergoing replacement.

The second characteristic is that there is almost no day-to-day pollution during operation (setting aside the major issue of nuclear waste, which we will deal with in due course). Other than electricity and exhaust heat/steam, a nuclear power station produces little more than a typical office block does. The only carbon dioxide and waste products come from the human activity within. Undetectable trace quantities of radioactive hydrogen are released over time.
These factors form an important part of the case for nuclear power. No pollutants or greenhouse gases are emitted into the air, so the power generated is extremely clean and speaks to the issue of climate change. The fuel can be sourced from around the world, much of it from countries that are of a friendlier disposition, and energy can be saved by significantly reducing the amount of transportation required – a year’s worth of nuclear fuel can be moved in a few truckloads. The high density enables practical stockpiling of years of fuel, keeping prices stable and avoiding economic effects. These are the reasons why – unlike Germany – France has lower carbon emissions and is not facing a major cost of living crisis, because 70% of its electricity comes from nuclear power.
In the next article, I’ll talk about the dangerous side of nuclear power : the major accidents and the problems posed by the need for waste disposal.
centre-leftish waffler working in IT and living in Belfast
Alliance, but writing in a strictly personal capacity.
Discover more from Slugger O'Toole
Subscribe to get the latest posts to your email.
